Transport System in Plants
We know that the roots of a plant absorb food, water and mineral salt from the soil and conduct these up by way of the stem to the leaves where the manufacture of organic food takes place.
You might also know that the organic food (mainly carbohydrates) prepared by the green cells of the leaf are conducted to the branches, stem, and roots for their use and storage.
Transport of Materials in Plants
This function of conduction or transport is carried out in specialized groups of cells known as the xylem and phloem which, with other types of cells, form the vascular bundles of the roots, stem, and leaves.
How do Food and Water Transport in Plants?
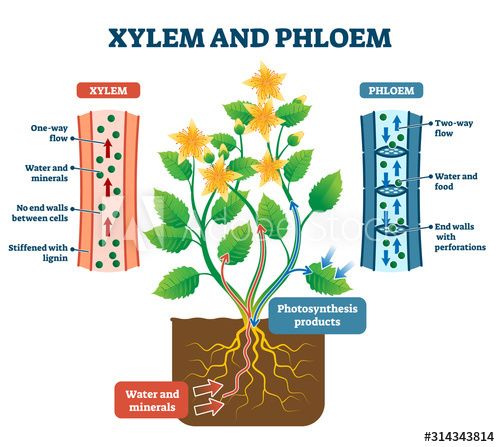
Xylem and Phloem
The xylem tissue, composed mostly of dead cells, is chiefly concerned with the upward transport of water and mineral salts.
However, it has been shown that the phloem tissue may also conduct mineral salts depending on the plant, the region of the plant, and the type of mineral salt concerned.
The transport of synthesized food material in all directions is via the living phloem tissue.
Osmosis and Diffusion
The absorption and transport of materials in plants involve two important physical processes – osmosis and diffusion.
These processes occur throughout the entire plant body.
In order to understand how the living cells of plants carry out their functions, knowledge of osmosis and diffusion is necessary.
Osmosis
When two solutions of different concentrations are placed on either side of a semi-permeable membrane, the solvent (usual water) from the less concentrated solution flows into the solution of higher concentration through the separating membrane until both solutions are of equal concentration, that is, isotonic.
The movement of the solvent from the solution of lower concentration to that of higher concentration through the semi-permeable membrane is known as osmosis.
A semi-permeable membrane is one that allows certain types of substances to pass through and not others, depending on the size of the molecules of which the substances are composed.
In a situation where osmosis occurs, the solution of higher concentration is said to have a higher osmotic pressure than the other solution.
As osmosis proceeds, there tends to be an equalizing of osmotic concentrations and pressures.
Osmosis in Plants
The epidermal cells of roots and the single-celled root hairs are in constant contact with soil water which is found in the spaces between the soil particles.
Soil water contains a number of mineral salts in solution.
As I’ve said earlier, the plant cell has a permeable cell wall which allows most substances to pass through into the cell.
However, the cytoplasm at the cell boundaries acts as a semi-permeable membrane and allows only certain substances to pass through it.
The vacuole of the cell contains cell sap which is a solution containing dissolved sugar and mineral salts.
It has a higher osmotic concentration than the soil water and consequently, exerts a higher osmotic pressure.
Therefore, the sol water passes into the vacuole by osmosis and thereby dilutes the cell sap.
In doing so, it reduces its osmotic concentration as well as its osmotic pressure.
Some of the water and dissolved substances in the cell sap then flows by the process of osmosis into the vacuoles of the neighboring cells where the osmotic concentrations and pressures are relatively higher.
It is also possible for water and dissolved substances to pass from the cytoplasm of one cell (with lower osmotic pressure) to another (with higher osmotic pressure) without passing through vacuoles.
Diffusion
The diffusion of gases, liquids or solutes, refers to the movement of the molecules of these substances from one place to another.
When two gases or liquids are placed in the same vessel, the two types of molecules move about and mix with one another. this mingling is known as diffusion and is quite different from the phenomenon of osmosis.
Diffusion usually takes place from a region of higher concentration to one of lower concentration.
In plants, generally, water vapor diffuses outwards and gases (mainly oxygen and carbon dioxide) diffuse in both directions between the plant organs and the surrounding atmosphere.
Turgidity and Flaccidity
When water enters the vacuole of a cell, the volume of the cell sap increases.
The pressure exerted by the contents of the cell against the rigid cell wall also increases.
This pressure is known as turgor pressure. The cell wall resists the expansion of the cell contents by pushing inwards with a pressure equal in magnitude but opposite in direction to the turgor pressure.
This pressure is sometimes referred to as wall pressure. When the cell wall is fully stretched, that is, when the pressure exerted by the contents of the cell outwards is equaled by the resistance of the cell wall inwards, the cell is said to be turgid.
In contrast to turgidity, that is, the state of a cell being turgid, cells sometimes suffer a loss of water which causes them to become weak, limp, and soft.
This condition is known as flaccidity usually occurs when plants lose water to the atmosphere faster than it can be obtained from the soil, so that water from the vacuoles is withdrawn.